Background
TP53-mutated (m) acute myeloid leukemia (AML) is an adverse risk disease, which continues to have dismal outcome in the contemporary era of novel therapies. Venetoclax (VEN) in combination with hypomethylating agents (HMA; azacitidine or decitabine) have shown to improve complete remission (CR) rates compared to HMA alone, however no significant differences in overall survival (OS) have been observed.
Methods
We conducted a retrospective study through the COMMAND consortium (a collaboration of acute leukemia experts from 10 US academic institutions) to analyze the impact on survival outcomes of HMA plus (+) VEN compared to HMA-based therapies in adult (≥18 years) pts with TP53m AML. The AML Database of 381 pts with TP53m AML diagnosed between 2012-2022 was queried and 154 (40%) (HMA [n=50] and HMA +VEN [n=104]) pts were identified eligible for this analysis. Continuous variables were summarized as median (range) while categorical variables were reported as frequency (percentage). The Kaplan-Meier method was used to estimate OS, defined as time from diagnosis to death or last follow-up. Logistic regression models were used to determine the univariate and multivariate of OS. Multivariable models included all significant univariate predictors. All tests were two-sided with a p value <0.05 considered statistically significant.
Results
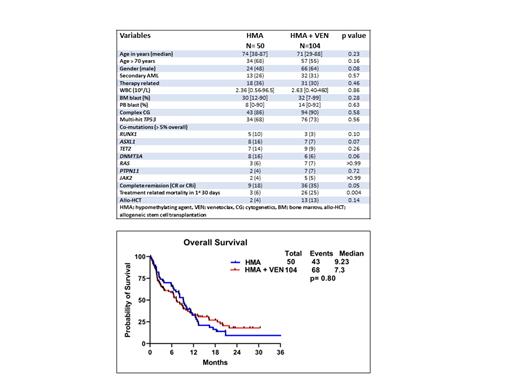
Baseline characteristics and responses
The median age (years [yrs]) was 74 (range [R], 38-87) and 71 (R,29-88) yrs in the HMAand HMA + VEN groups (gps) (p= 0.23), respectively ( Table 1). Thirty-four (68%) and 57 (55%) patients (pts) were ≥ 70 yrs in the HMA and HMA + VEN gps, respectively (p= 0.16). Twenty-six percent vs 31% ( p=0.57), 86% vs 90% ( p=0.58) and 68% vs 73% ( p=0.56) of pts had secondary AML, had complex cytogenetics (CG), and had multi-hit TP53m in the HMA and HMA + VEN gps, respectively. The proportion of pts with most frequently occurring (> 5%) myeloid co-mutations ( RUNX1, ASXL1, TET2, DNMT3A, RAS, PTPN11 and JAK2) were not significantly (NS) different between the HMA and HMA + VEN gps, as shown in Table 1. The early treatment related mortality in first 30 days of induction was higher in the HMA+VEN gp (25%) when compared with the HMA gp (6%), p=0.004. The complete remission rates (CR/CRi) were higher in the HMA + VEN gp compared to the HMA gp (35% vs 18%, p=0.05). Similarly, the proportion of pts receiving allogeneic stem cell transplant (allo-HCT) after induction was higher in the HMA + VEN gp when compared with the HMA gp (13% vs 4%; p=0.14).
Duration of response and overall survival
The median follow up was 9.8 months (mo) (R, 1-56) and 5.80 mo (R, 1-30.4) in the HMA and HMA + VEN gps, respectively ( p=0.12). The median duration of response (DOR) was higher in the HMA +VEN gp (15.6 mo) when compared with the HMA gp (7.93 mo), but NS ( p=0.26). The median OS was NS different when comparing the HMA and HMA + VEN gps (9.23 vs 7.3 mo, p=0.80 [ Figure 1]).
Multivariable analysis for duration of response and overall survival
We did multivariable analysis (MVA) for DOR and OS using baseline variables ( Table 1) that showed significance/trend towards significance in univariate analysis ( p<0.1).
Allo-HCT (HR; 0.11, 95% CI: 0.02-0.52, p=0.005) retained significance for better DOR, whereas age ≥ 70 yrs (HR; 1.7, 95% CI: 0.63-4.65, p=0.28) and complex CG (HR; 3.05, 95% CI: 0.79-11.73, p=0.10) did not retain significance in MVA.
In MVA for OS, achievement of CR/CRi (HR; 0.35, 95% CI; 0.20-0.59, p=<0.001) and allo-HCT after induction (HR; 0.06, 95% CI; 0.09-0.46, p=0.007) retained favorable significance for OS, whereas complex CG (HR; 2.36, 95% CI: 1.11-4.99, p=0.02) retained significance for shorter OS. PTPN11m (HR; 1.57, 95%CI: 0.75-3.28, p=0.23) and TET2m (HR; 1.02, 95% CI: 0.56-1.87, p= 0.92) did not retain significance in MVA.
Conclusion
In this multi-center real-world study, while HMA+VEN led to improvement in CR rates and a higher proportion of pts were bridged to allo-HCT, it did not associate with an improvement in OS when compared with HMA monotherapy. More effective treatment strategies are warranted to improve outcome of pts with TP53m AML.
Disclosures
Atallah:Takeda: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding. Shallis:Curio Science: Consultancy; Gilead Sciences: Consultancy; Bristol Myers Squibb: Consultancy; Rigel: Consultancy; Servier: Consultancy. Patel:Pfizer: Research Funding; Kronos Bio: Research Funding; AbbVie: Honoraria; BMS: Honoraria. Goldberg:Trillium: Research Funding; AROG: Research Funding; Celularity: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Research Funding; DAVA Oncology: Honoraria; Abbvie: Consultancy, Research Funding; Astellas Pharma: Consultancy; Genentech: Consultancy; Aprea: Research Funding; Aptose: Research Funding; ADC Therapeutics: Research Funding; Prelude: Research Funding. Abaza:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Rigel: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Biomea: Research Funding; Curis: Research Funding; Biosight: Research Funding; ALX Oncology: Research Funding. Palmisiano:Genentech: Research Funding; Rigel: Consultancy; Abbvie: Consultancy, Research Funding. Kota:Incyte: Research Funding; Novartis: Honoraria; Kite: Honoraria; Pfizer: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal